| Courtesy of nynewsdaily.com |
Cell phones, flashlights,
smoke detectors, cars, and iPods are all enabled by a technology which we take
for granted in today’s age: batteries. We use them every day, but
what is it that makes batteries able to power the devices which illuminate the
dark, send messages across the globe, and alert us of unseen dangers?
While batteries come in
a wide variety of sizes and shapes, they all work by the same basic principle,
known as an electrochemical process. Originally discovered in 1799 by the
Italian physicist Count Alessandro Volta, the chemical reaction taking place
involves the flow of electrons between two materials immersed in an electrolyte.
More formally, these three parts are referred to as the anode (-), the cathode
(+), and the electrolyte. These symbols may appear familiar to you if you’ve
replaced a battery recently!
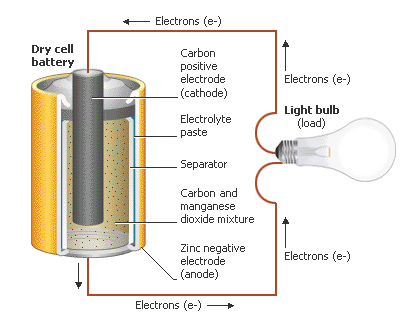 |
| Courtesy of http://media.tumblr.com/tumblr_loecuzgfyS1qf00w4.gif |
In the battery, the
anode and cathode, known as electrodes, are separated by a barrier which allows
electric charge to flow between the two electrodes but prevents the two metal
pieces from coming into contact. The medium through which the electrical charge
travels is the electrolyte, a solution or paste filled with charged particles. Each type of battery contains a specific electrolyte.
When a battery is placed
in a device, wires within the device now connect the anode and the cathode,
making a complete circuit for electrons to flow between the two electrodes. At
the anode, the metal surface loses electrons to form ions (charged atoms or
molecules) which combine with other ions from the electrolyte, in a process
known as oxidation (an example is given below in Equation 1).
Zn(s) --> Zn2+(aq) + 2e- Eq. 1
The liberated electrons
then decide to leave the negative and crowded party at the anode and make their
way to the much more upbeat and positive shin-dig going on at the cathode. It
is here that ions from the electrolyte combine with the released electrons in
a process known as reduction (an example is given below in Equation 2).
2MnO2 + 2e-
+ 2NH4Cl --> Mn2O3 + 2NH3 +
H2O + 2Cl- Eq. 2
 |
| Courtesy of Lonestarlearning.com |
A friendly pneumonic to
remember these principles is "LEO the lion goes GER" for Loss of Electrons is
Oxidation and Gain of Electrons is Reduction. The electrons produced from
reduction and oxidation are the same ones that power your devices!
Batteries eventually run out of
power because there are a limited number of ions in the electrolyte solution. Once the ions are depleted, the reduction-oxidation reactions which cause the
electrons to flow, can no longer occur. Rechargeable batteries work by running
a current of electrons into the battery, reversing the cathode and anodes, and
releasing ions back into the solution. Below are some of the common battery
types and the materials that make them up.
Battery
Type
|
Cathode
|
Anode
|
Electrolyte
|
Zinc-Carbon
|
Manganese
Dioxide (MnO2)
|
Zinc
|
Ammonium
Chloride (NH4Cl) or Zinc Chloride (ZnCl2)
|
Alkaline
|
Manganese
Dioxide (MnO2)
|
Zinc
Powder
|
Potassium
Hydroxide (KOH/H2O)
|
Lithium-Ion
|
Lithium
Cobalt Oxide (LiCoO2)
|
Carbon
(C)
|
Lithium
Hexaflurorphosphate (LiPF6)
|
Lead-Acid
|
Lead
Dioxide (PbO)
|
Lead
(Pb)
|
Sulfuric
Acid (H2SO4/H2O)
|
 |
| The Fisker Karma, an electric car (courtesy of Road & Track) |
So next-time you put
some new batteries into a device or charge your cell phone, think of all those
electrons speeding through the wires as ions are formed and destroyed in the
tug of war between cathode and anode. Who knew batteries could be so interesting!
References:
- http://electronics.howstuffworks.com/everyday-tech/battery.htm
- http://www.qrg.northwestern.edu/projects/vss/docs/power/2-how-do-batteries-work.html
- http://en.wikipedia.org/wiki/Battery_(electricity)
- http://www.tumblr.com/tagged/how+do+zinc+carbon+batteries+work
No comments:
Post a Comment